AQA GCSE Atomic Structure and the Periodic Table Revision EdPlace's AQA Single Subject GCSE activities, exam style questions and practice papers covers content from GCSE Biology (8461), GCSE Chemistry (8462) and GCSE Physics (8463) specifications EdPlace's GCSE examstyle questions and practice papers help your child hone their exam Periodic Table With Names And Atomic Mass And Number We should always learn something new to download the Periodic Table With Names And Atomic and know the elements or chemicals present on our earth There are 118 chemical elements in the periodic table If you learn the elements and their properties separately, then it won't be very clearThe work he published in 1869 is the basis of the modern Periodic Table today There were a number of important features of the Periodic Table proposed by Mendeleev • He listed the elements in order of increasing atomic mass (or weight as it was known then) but he

Putting Electrons In Shells
Gcse periodic table with mass and atomic numbers
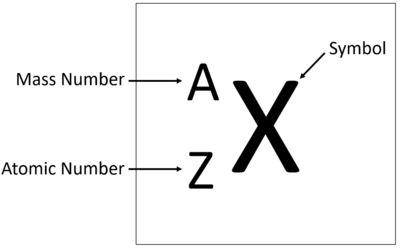
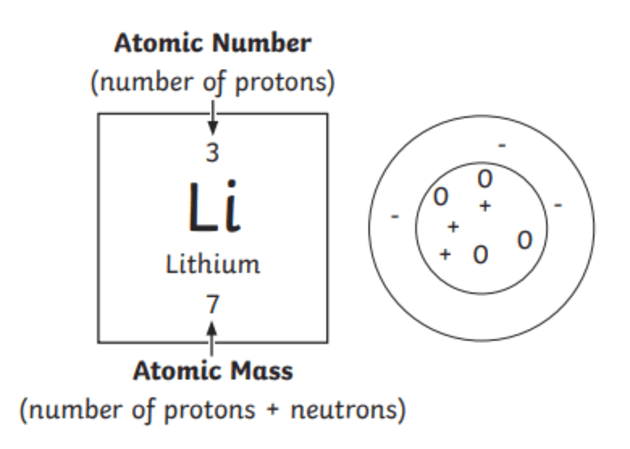
Gcse periodic table with mass and atomic numbers-Use the mass number and atomic number to calculate the number of protons, neutrons and electrons in an atom or ion Relate the size of atoms to objects that can be seen 116 Relative atomic mass State what relative atomic mass is and how it is calculated Calculate relative atomic mass from data given 117 Electronic StructureThe mass number counts protons neutrons (19) If there are 9 protons, there must be 10 neutrons for the total to add up to 19 The atomic number is tied to the position of the element in the Periodic Table and therefore the number of protons defines what sort of element you are talking about




Periodic Table Without Names
The data collected from a mass spectrum can help to calculate a relative atomic mass RAM = Σ (isotopic mass x %Mendeleev arranged the elements in his periodic table in order of increasing atomic mass he also left gaps in the table to make sure that elements with similar properties stayed in the same groups when new elements that were discovered fitted into those gaps his theory was further provenQuestion 1 State the principle of conservation of mass Question 2 Calculate the relative atomic mass (A r) of chlorine which is found to contain 75% of atoms with mass number 35, and 25% of atoms with mass number, 37 Question 3 Calculate the relative formula mass (M r)of the following substances NH 4 Cl (Ammonium chloride);
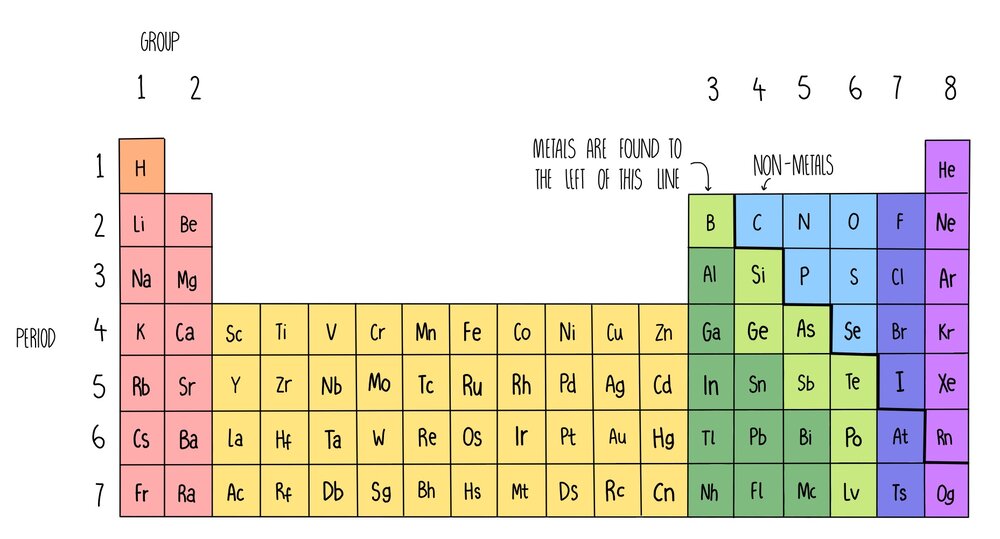
The periodic table below is based on the ones used by the different examination boards The group numbers 1 to 0 (the top ones) are used in most GCSE courses The group numbers 1 to 18 were recommended by IUPAC in 19 At the moment these are only used in OCR courses There is a summary at the bottom of the page It shows the differencesThe periodic chart sorted by Atomic Mass Name chemical element Symbol Atomic numberNumber of electrons = atomic number
Atomic number, mass number and isotopes Atoms contain protons, neutrons and electrons The electrons are arranged in shells around the nucleus The periodic table is aMass number _____ number Where possible use full sentences to answer this question 21a) A Periodic Table entry for sodium is shown below, fill in the black space on the labelling b) Calculate the number of protons, neutrons and electrons in an atom of sodium _____(1 mark) Q6 Fill in this table for the relative masses of parts of the atom Name of particle Relative mass Proton Neutron



Gcse Additional Science For The 21st Century Topic C4 Chemical Patterns




Updated Periodic Table Printable Resource Rsc Education
C 22 Electronic structure and the periodic table AQA GCSE Chemistry C2 The Periodic Table Kerboodle Answers Page No 25 1a Periodic means that the elements in the periodic table shows patterns in electronic configuration, physical and chemical properties and repeat itself with increasing atomic numberStudents should be able to Calculate the numbers of protons, neutrons and electrons in an atom or ion, given its atomic number and mass number Relate size and scale of atoms to objects in the physical world MS 1d WS 43 Use SI units and the prefix nano MS 1b Recognise expressions in standard form 4116 Relative atomic massAQA GCSE Atomic Structure and the Periodic Table practice papers EdPlace's AQA Single Subject GCSE activities, exam style questions and practice papers covers content from GCSE Biology (8461), GCSE Chemistry (8462) and GCSE Physics (8463) specifications EdPlace's GCSE examstyle questions and practice papers help your child hone their exam



Made Easy Chemistry How To Calculate Relative Atomic Mass



Element Key Stage Wiki
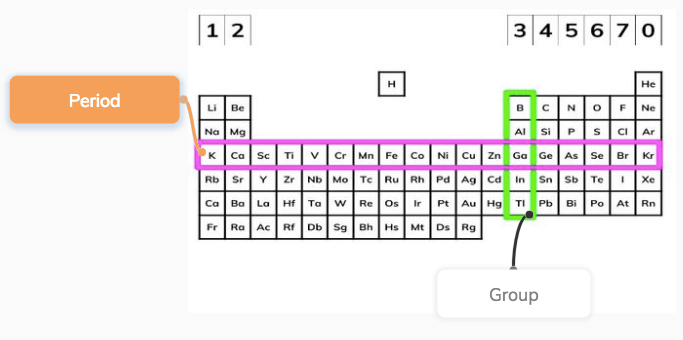
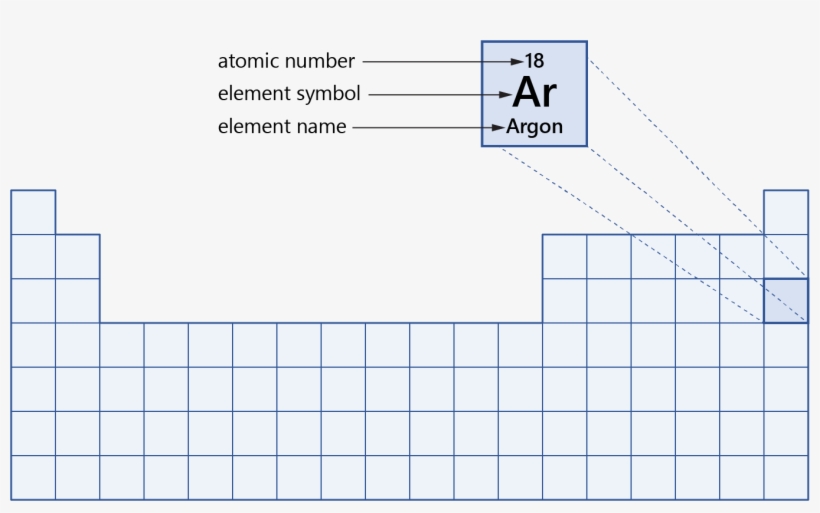
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H With a standard atomic weight of circa 1008, hydrogen is the lightest element on the periodic table Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic massAs you can see, for this particular table (some look a LITTLE different), the atomic mass for each element is found at the top of each box, and the atomic number at the bottom For all periodic tables, each box represents one atom Groups and Periods You may notice that the table is labelled, with groups across the top and periods along the sideThe periodic table is a fundamental part of chemistry No wonder then that it is studied in GCSE science!




A Gcse Igcse Science Chemistry Multiple Choice Quiz On The Periodic Table Easier Version At Doc Brown S Chemistry




Boardworks Gcse Additional Science Chemistry Ppt Video Online Download
GCSE Periodic table Keyword Definition Atomic mass the number of protons and neutrons in the atom Atomic number the number of protons in the nucleus of an atom Groups A group is a column of elements in the periodic table of the chemical elements Periods In each period (horizontal row), the atomic numbers increase from left to rightAtomic Number – Protons, Electrons and Neutrons in Iridium Iridium is a chemical element with atomic number 77 which means there are 77 protons in its nucleusTotal number of protons in the nucleus is called the atomic number of the atom and is given the symbol ZThe total electrical charge of the nucleus is therefore Ze, where e (elementary charge) equals to 1,602 x 1019Their relative atomic mass How did Mendeleev organize his periodic table?
%252C_black_and_white.png?revision=2)



3 4 Atomic Mass And Atomic Number Chemistry Libretexts




Gcse Chemistry 1 9 How Are Atomic Number And The Periodic Table Linked Youtube
Al 2 (SO 4) 3 (Aluminium119 rida The number of protons in the nucleus is called the atomic number The atomic number ofThis modern periodic table lists elements according to their atomic number, if they were arranged according to atomic mass potassium and argon would be the wrong way round Elements having the same number of electrons in their outermost shell are placed in vertical columns called groups They have similar chemical properties From left to right, across each horizontal row (period) of




How To Read The Periodic Table 14 Steps With Pictures Wikihow



What Is Atomic Structure Answered Twinkl Teaching Wiki
Eti Report of a Sustaining Improvement Inspection in May 18 The report can be downloaded by clicking the link below HereKnowIT questions – AQA GCSE Atomic Structure & the Periodic Table better hope − brighter future A Atoms, Elements, Compounds and Mixtures part 1 – Atoms, Elements, A silver atom with atomic number47 and mass number 108 b) KnowIT questions – AQA GCSE Atomic Structure & the Periodic Table better hope − brighter futureGCSE Chemistry Paper 1 Atomic Number and Mass Number In this GCSE Chemistry video we look at the atomic number and the mass number We learn how the atomic number and mass number tell us about the particles in an atom We then



The Element With Atomic Number 113 Has Just Been Discovered Into Two Different Forms One With 139 Neutrons And One With 145 Neutrons If The First Form Occurs 75 Of The Time



Periodic Table
Mass spectrometers can be used to determine all the isotopes present in a sample of an element and their percentage or relative abundancy; Periodic Table with Atomic Mass and Atomic Number You will get blank space on every box to fill in the elements number, atomic weight, atomic mass, charge, etc It will help them in better learning as well as practicing We know that practice makes everybody perfect, so they can quickly solve any chemistry problems if the students practiceThe atomic number therefore tells you what the element is (see the periodic table) What is the Mass Number?




Pin On Class Is In Session




Periodic Table Elements Wild Country Fine Arts
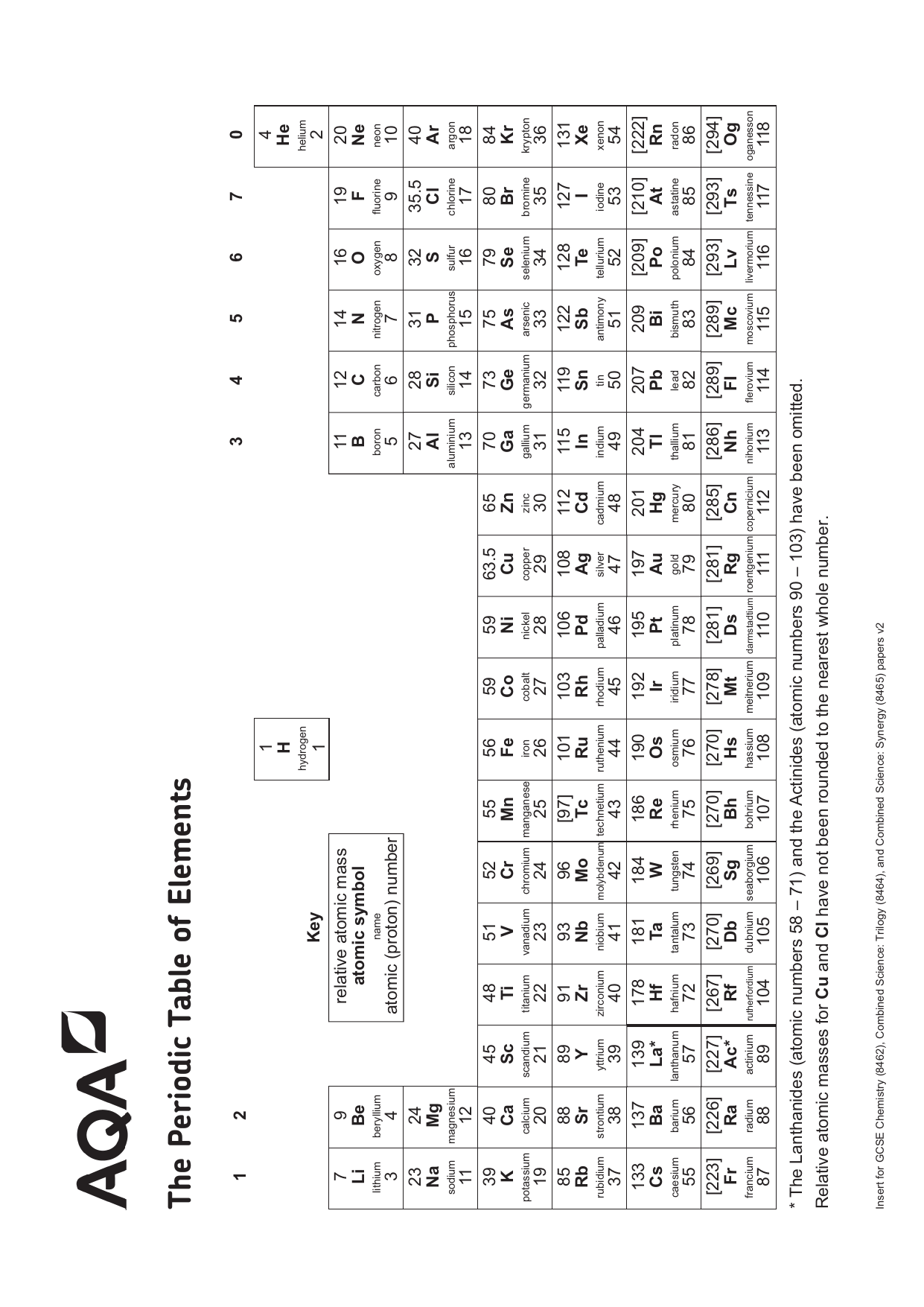
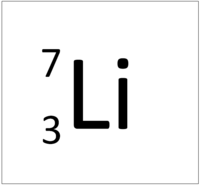
The Periodic Table of Elements 7 Li lithium 3 23 Na sodium 11 39 K potassium 19 85 Rb rubidium 37 133 Cs caesium 55 223 Fr 87 * The Lanthanides (atomic numbers 58 – 71) and the Actinides (atomic numbers 90 – 103) have been omitted Relative atomic masses for Cu and Cl have not been rounded to the nearest whole number francium 12 Be Key 9 beryllium 4The periodic table, also known as the periodic table of (the) elements, is a tabular display of the chemical elementsIt is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistryIt is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit a periodic dependence on their atomic numbers full size periodic table of elements with atomic mass and valency › pdf A Gcse Igcse Science Chemistry Multiple Choice Quiz On The 71 Tutorial Periodic Table Atomic Number 53 With Pdf And Video Valency Chart Of First 30 Elements Barta Innovations19 Org




Periodic Table Mass Number Atomic Mass Atomic Number Symbol Chemical Element Text Plan Png Pngwing




Atomic Structure And The Periodic Table Contents Page
The Periodic Table Mendeléev recognized that in order for the elements to be grouped in patterns he sometimes needed to change the order of the atomic masses It was not until work carried by Moseley in 1913 that the atomic number of the elements were identifiedThe total mass of one atom of the given element Its unit is called the unified atomic mass unit and is denoted by the symbol 'u' Standard atomic weight is used to give the value of the mean of the atomic masses in a mixture of isotopes in a given sample of an element Atomic Mass of First 30 Elements Given below is a table that lists the first 30 elements based on atomic number and their corresponding atomic massThe relative atomic mass quoted on the periodic table is a weighted average of all the isotopes of an element;




Periodic Table With Atomic Mass



What Is The Periodic Table Definition From Seneca Learning
The number of protons plus the number of neutrons is called the mass number Sodium has 11 protons and 12 neutrons The mass number is 11 12 = 23 The mass number is the total number of particles in the nucleus The mass number and the atomic number are writtenFirst Elements On The Periodic Table Crossword Wordmint 1 to elements of periodic table with its atomic number mass and list of first elements with atomic mass symbol number what are the first elements in periodic table quora structure of an atomThe atomic number counts the number of protons (9);




Free Printable Periodic Table Chart Science Notes And Projects Periodic Table Words Periodic Table Periodic Table Of The Elements
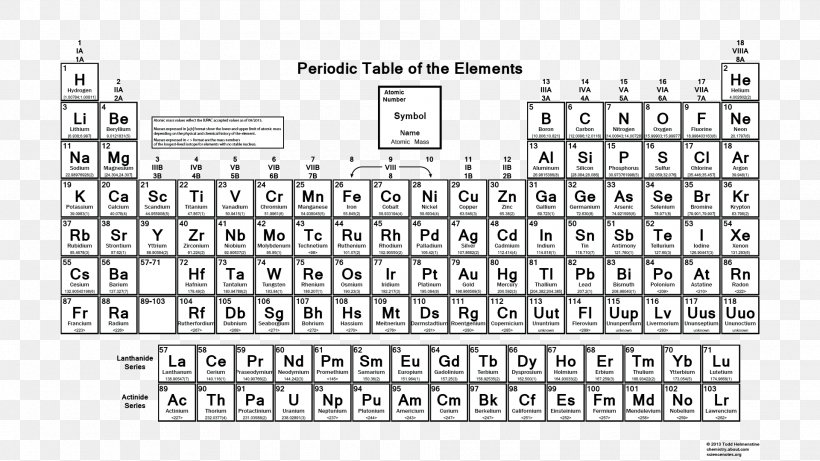



Periodic Table Atomic Mass Atomic Number Chemistry Png 19x1080px Periodic Table Area Atom Atomic Mass Atomic
Both the atomic number and the mass number are given on the Periodic Table but it can be easy to confuse them Think MASS = MASSIVE, as the mass number is always the bigger of the two numbers, the other smaller one is thus the atomic / proton number The Basis of the Periodic TableGCSE Atomic Structure Revise Elements' Mass and Number KS1 (Age 57) KS2 (Age 711) 11 (Age 711) KS3 (Age 1114) GCSE (Age 1417) Spanish ESL Games Cup of Tea PSHE Aluminium has 3 electron shells with 3 electrons in the outer shell Atomic Structure 2The mass number this is the total number of protons and neutrons



Pass My Exams Easy Exam Revision Notes For Gsce Chemistry




Developing A Modern Periodic Table From Spirals To The Stars Science Museum
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How works Test new features Press Copyright Contact us CreatorsThis slideshow covers the new AQA 16 GCSE content on Chemistry unit 1, including the structure of the atom, elements, compounds, balancing equations, naming compounds, mixtures, filtration, evaporation, distillation, chromatography, the development of atomic structure theory, Rutherford scattering, mass and atomic number, isotopes, electron structure, the periodic table, how the periodic tableStandard Atomic Weight The standard atomic weight is the average mass of an element in atomic mass units ("amu") Though individual atoms always have an integer number of atomic mass units, the atomic mass on the periodic table is stated as a decimal number because it is an average of the various isotopes of an element



Periodic Table Creative Chemistry
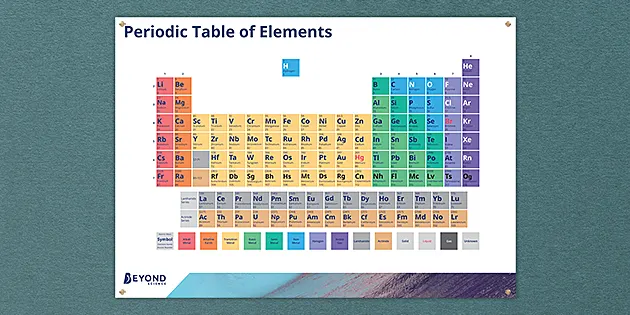



Periodic Table Of Elements Poster Ks3 Chemistry Beyond
This exciting, interactive AQA Syllabus A quiz was written by teachers specifically to help Year 10 and Year 11 pupils revise the order and types of element found within the periodic tableQ3 Magnesium's atomic number is 12 How many protons and electrons does it have?Found on the left hand side of the periodic table, make up about 80 % of the elements Relative atomic mass Average mass of atoms of an element taking into account mass




Atomic Structure The Periodic Table Cie Igcse Chemistry Revision Notes




The Periodic Table D Atomic Number Png Image Transparent Png Free Download On Seekpng
Protons Electrons (2 marks) Q4 Label the diagram (3 marks) Size and mass of atoms Q5 Where is almost all the mass in an atom?Relative atomic mass (symbol A r) or atomic weight is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constantThe atomic mass constant (symbol m u) is defined as being 1 / 12 of the mass of a carbon12 atom Since both quantities in the ratio are masses, the resulting value is A periodic table made with the the relative atomic mass at the top and the atomic (proton) number listed at the bottom Useful for the radioactivity component of the Physics course for writing equations describing alpha and beta decay of atoms




Atomic Number Mass Number Properties Of Matter Chemistry Fuseschool Youtube



3
The atomic masses in Table 211 "The Basics of the Elements of the Periodic Table" represent the number of decimal places recognized by the International Union of Pure and Applied Chemistry, the worldwide body that develops standards for chemistry The atomic masses of some elements are known very precisely, to a large number of decimal places Therefore, every student deserves outstanding teaching, no matter where they live, where they go to school, or how much money they have 91 GCSE Chemistry Paper 1 Atomic Structure and theBoth the atomic number and the mass number are given on the periodic table, but it can be easy to confuse them Think MASS = MASSIVE, as the mass number is always the bigger of the two numbers, the other smaller one is thus the atomic / proton number




Size Mass Of Atoms Aqa Gcse Chemistry Revision Notes




Gcse Periodic Table Introduction Worksheet Questions On Basic Ideas Of Its Structure Igcse Ks4 Science Revision Questions
H 2 SO 4 (Sulfuric acid); Elements on the Periodic Table are written with their Chemical Symbols and two numbers representing their Relative Atomic Mass and Atomic Number The Relative Atomic Masses on the Periodic Table are an average atomic massTo calculate the numbers of subatomic particles in an atom, use its atomic number and mass number number of protons = atomic number;




Putting Electrons In Shells




Free Printable Periodic Tables Pdf And Png Science Notes And Projects
Science Atomic Number Chart Barta Innovations19 Org Periodic Table Chemical Element Chemistry Atomic Number Molar Mass Periodic Table Of 118 Elements With Atomic Mass And Atomic Number 6 3 Periodic Law Chemistry Libretexts Color Periodic Table Of The Elements Atomic MassesOn the Periodic Table you will find every element that we know about, which in turn is a list of every atom that we have discovered Next to each element's symbol you will find several numbers The atomic number this is the number of protons;




Periodic Table Without Names
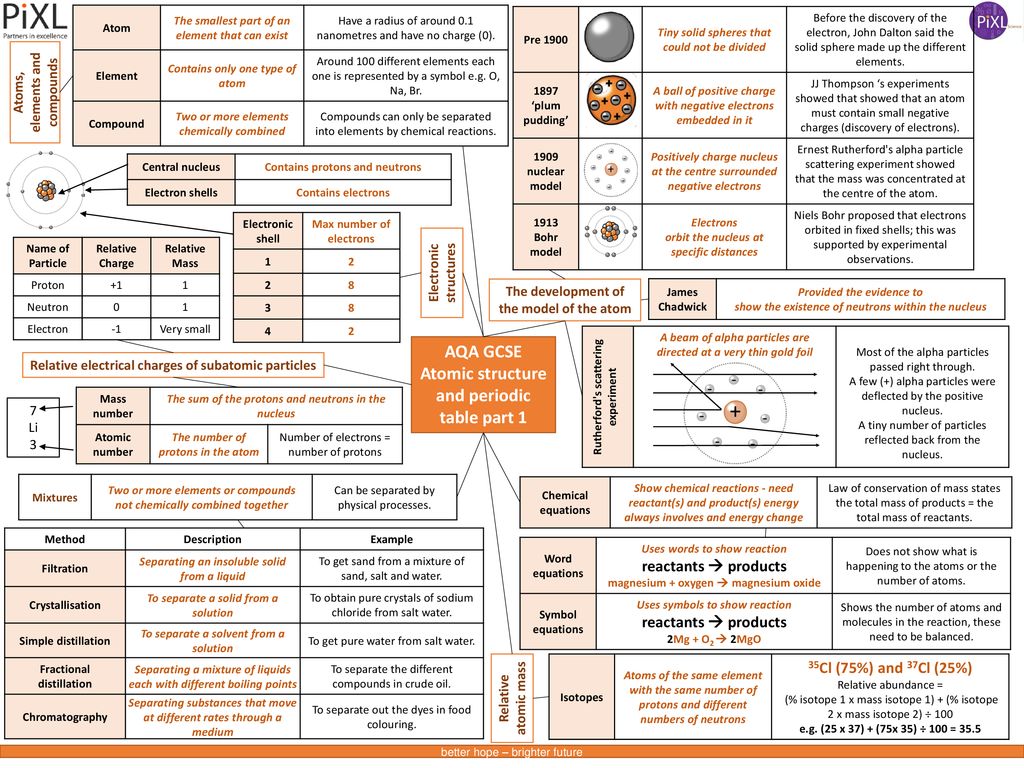



Aqa Gcse Atomic Structure And Periodic Table Part 1 Ppt Download




1 01 Elements And Compounds Sjp1618gcsechem




How To Read The Periodic Table Teaching Chemistry Teaching Middle School Science Chemistry Classroom



The Periodic Table Skoolers Com Csec Cxc Exam Preparation
/PeriodicTableoftheElements-5c3648e546e0fb0001ba3a0a.jpg)



Color Periodic Table Of The Elements Atomic Masses




Tennessine Wikipedia
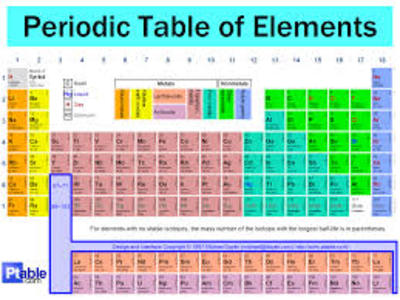



What Is An Atom Like P2 5 1 Aqa Gcse Physics Quiz




Atoms S Cool The Revision Website
:max_bytes(150000):strip_icc()/GettyImages-1147786242-dc7b08b722394905ad7cd734cdd76a93.jpg)



How To Use A Periodic Table Of Elements



Periodic Table Gcse Science Marked By Teachers Com




What Are Isotopes How Do They Affect Ram Chemistry Made Simple



Relative Atomic Mass Key Stage Wiki




Color Periodic Table With Shells Gcse Periodic Table With Mass And Atomic Numbers Png Image Transparent Png Free Download On Seekpng




Relative Atomic And Molecular Mass Ppt Download




Relative Atomic Mass A Question Science By Degrees



1




Atomic Structure Gcse The Science Hive




Atomic Structure Chemistry Gcse Revision
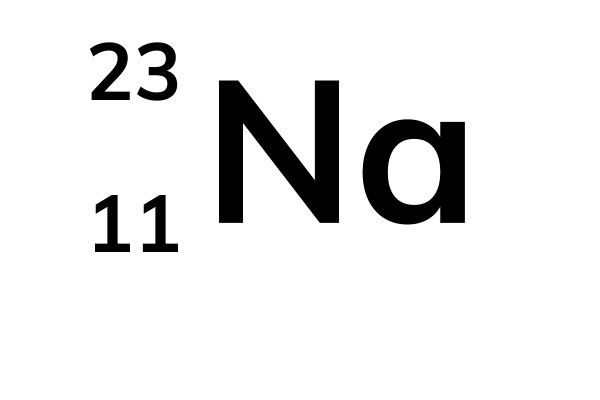



What Is Atom Size Number Definition From Seneca Learning
:max_bytes(150000):strip_icc()/PeriodicTableWallpaper-56a12d103df78cf7726827e81-0c02b1ee4c8b42478b651c7c9aaac7f9.jpg)



How To Use A Periodic Table Of Elements




Periodic Table With Relative Atomic Masses Relative Atomic Mass Periodic Table Periodic Chart




19 The Year Of The Periodic Table Rick Anderson



The Periodic Table Aqa The Science Hive




The Modern Periodic Table With Alkali Metals Transition Metals Halogens And Noble Gases Highlighted Periodic Table Picture Periodic Table Gcse Chemistry




Ocr The Periodic Table Is An Essential Tool For All Chemists It Is Full Of Useful Information Relative Atomic Mass Atomic Number Symbol Helium Ppt Download



1



Periodic Table Cell Atomic Number Element Name Symbol Diagram Free Transparent Png Download Pngkey




Atomic Structure The Periodic Table Cie Igcse Chemistry Revision Notes




Gcse Chemistry The Periodic Table Ocr 9 1 Youtube




Igcse Chemistry 1 14 Deduce The Electronic Configurations Of The First Elements From Their Positions In The Periodic Table




C Atomic Structure Igcse Chemistry Revision




Color Periodic Table With Shells Gcse Periodic Table With Mass And Atomic Numbers Full Size Png Download Seekpng
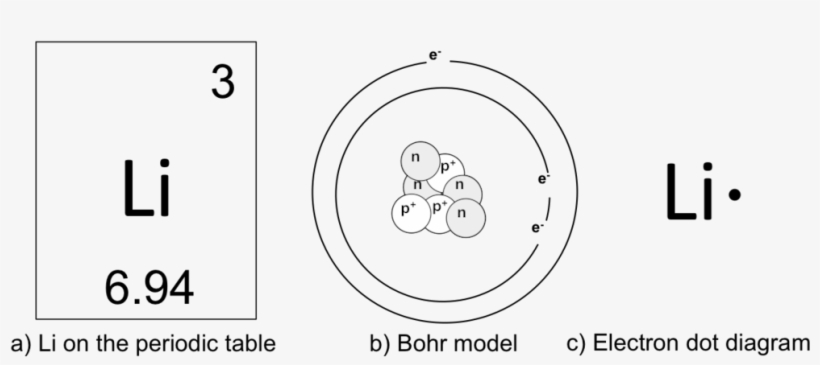



Elements Drawing Periodic Table Atom With Atomic Number 3 Png Image Transparent Png Free Download On Seekpng




Structure Of The Periodic Table Periodic Table Atomic Structure Chemistry Year 10 Gcse Diagram Quizlet




The Periodic Table Gcse Revision Chemistry Periodic Table Periodic Table 0 Revision World
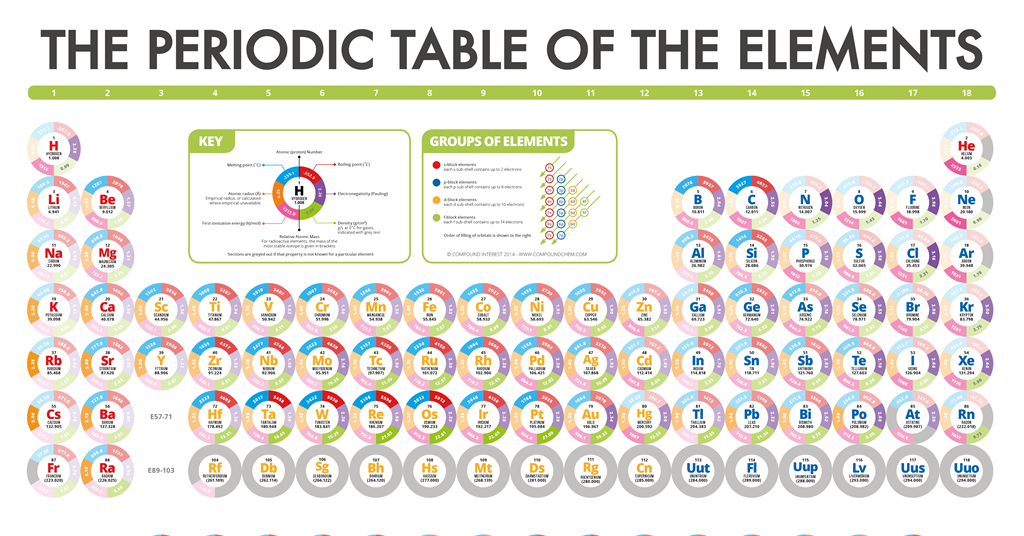



Elements Infographics Resource Rsc Education



Wikipedia Talk Wikiproject Elements Archive 12 Wikipedia




The Periodic Table Atomic Structure And The Periodic Table Chemistry Gcse 9 1 Flashcards Quizlet
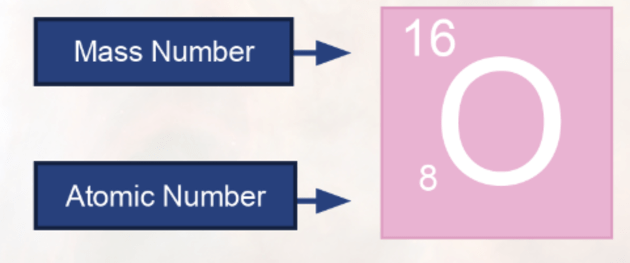



What Is An Element Answered Twinkl Teaching Wiki



Http Www Lordwilliams Oxon Sch Uk Force Download Cfm Id 4859




19 The Year Of The Periodic Table Rick Anderson



1




1 Historical Introduction To The Periodic Table History Origin Of Elements




Atomic Structure The Periodic Table Cie Igcse Chemistry Revision Notes




Periodic Table Atomic Structure Youtube



Swot Revision




Table Showing The Diagram Electron Configuration And Periodic Table Group Of Fluorine Neon Sodium And Calciu Electron Configuration Chemistry Gcse Chemistry



Atomic Number And Mass Number Gojimo
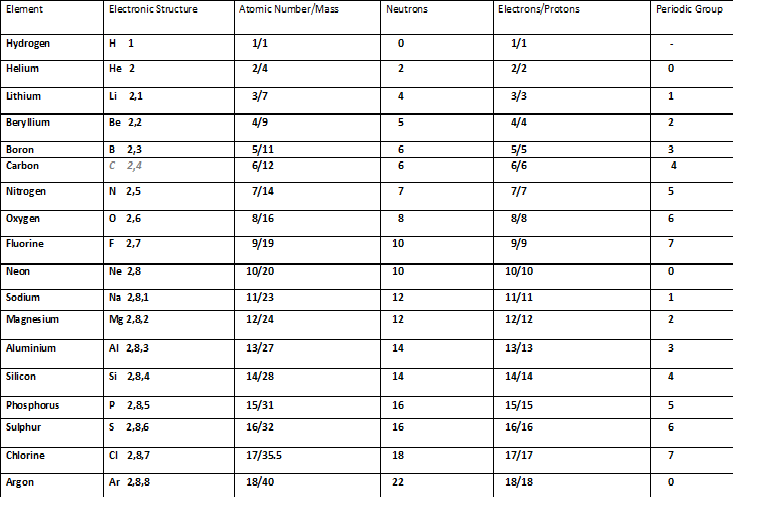



Atomic Number Nygma Science



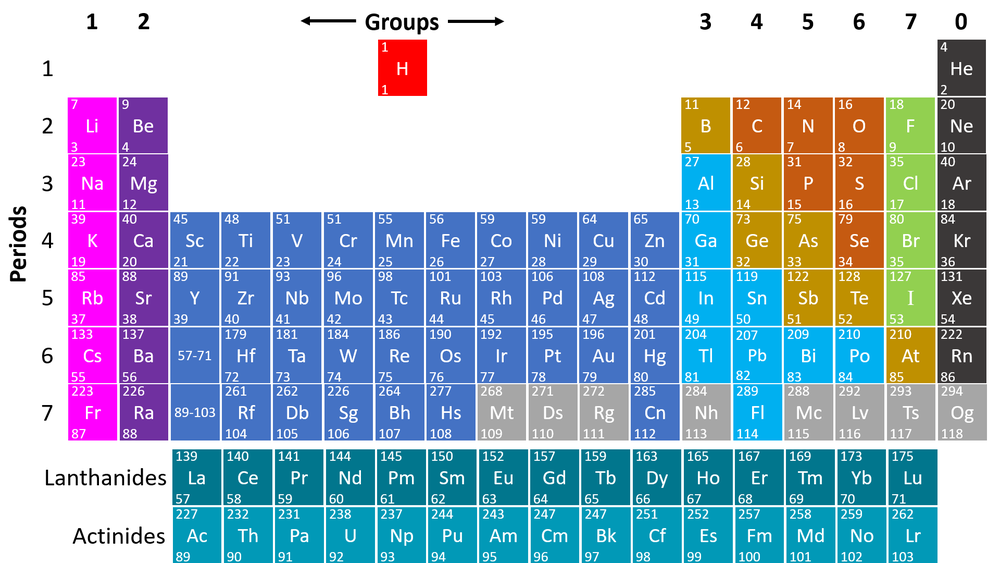
How Would You Find Molar Mass Example




Atomic Structure The Periodic Table Cie Igcse Chemistry Revision Notes




Atomic Number Key Stage Wiki




Boardworks High School Science Atomic Number And Mass Number Ppt Download



Pass My Exams Easy Exam Revision Notes For Gsce Chemistry




Gcse Science Revision Chemistry Relative Atomic Mass Youtube




History Development Of The Periodic Table



Iypt Activities History Of The Periodic Table Resource Rsc Education



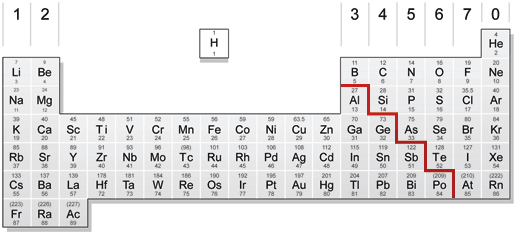
Periodic Table For Physics Radioactivity Atomic Mass Listed At The Top Teaching Resources




67 Periodic Table Of The Elements Ideas Periodic Table Of The Elements Periodic Table Science Notes




Gcse Chemistry 1 9 How Are Atomic Number And The Periodic Table Linked Youtube




67 Periodic Table Of The Elements Ideas Periodic Table Of The Elements Periodic Table Science Notes



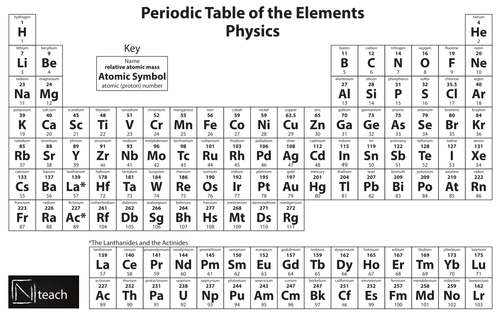
Periodic Table Key Stage Wiki




Which Elements Of The Periodic Table Forming Covalent Bonds Relate To Psition And Electron Configuration Doc Brown S Chemistry Revision Notes




C1 1 Atoms Secondary Science 4 All
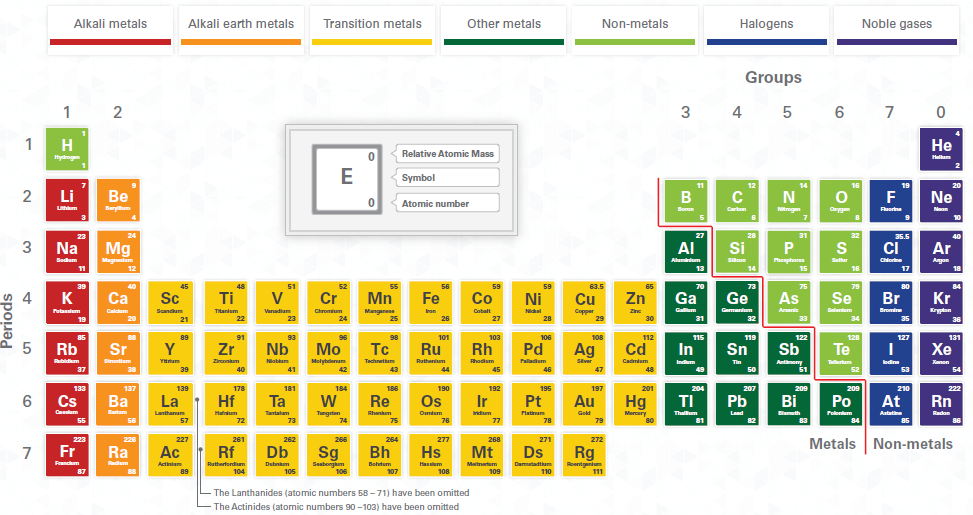



19 The Year Of The Periodic Table Rick Anderson
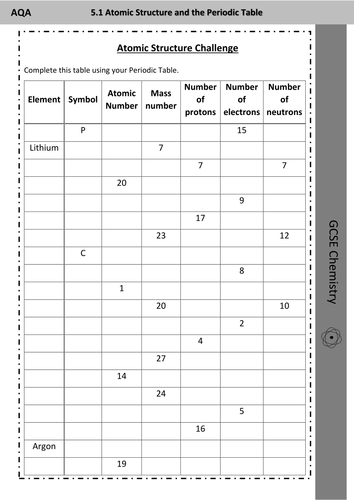



Gcse Atomic Structure Challenge Worksheet Determining Numbers Of Protons Electrons And Neutrons Teaching Resources
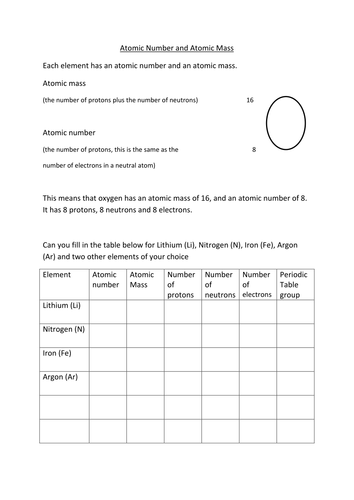



Atomic Mass And Atomic Number Teaching Resources




C1 1 The Periodic Table Secondary Science 4 All
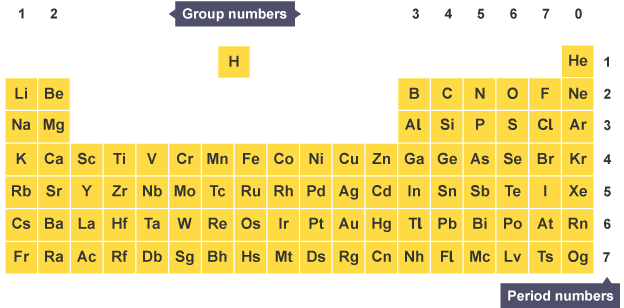



19 The Year Of The Periodic Table Rick Anderson
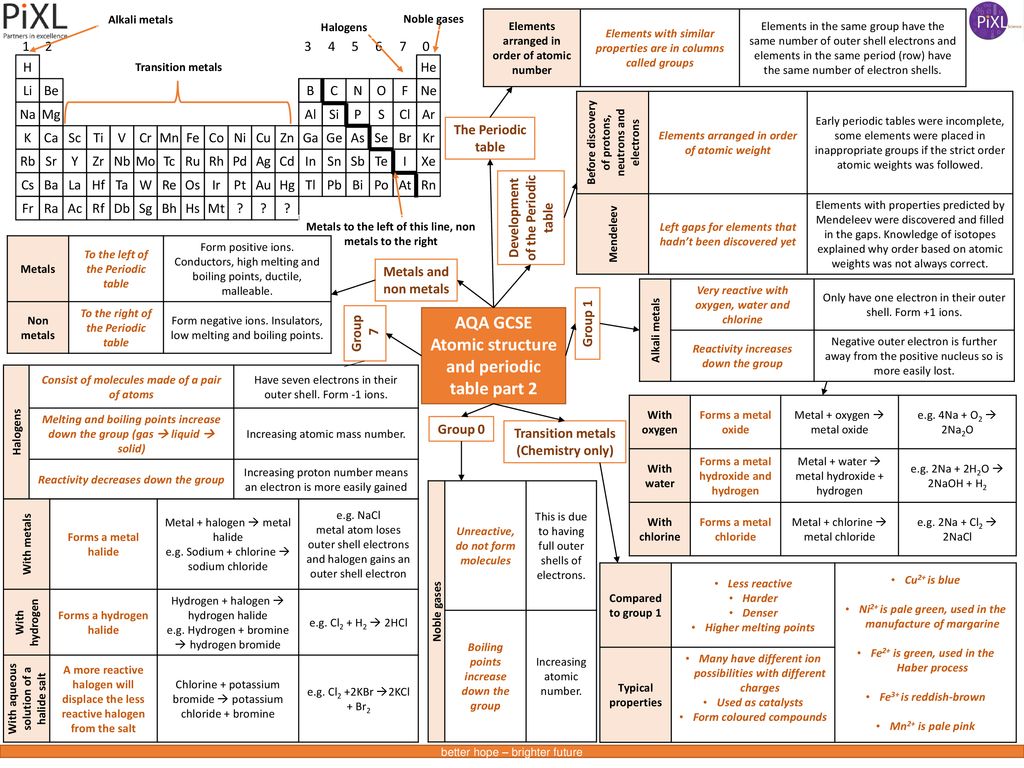



Aqa Gcse Atomic Structure And Periodic Table Part 1 Ppt Download




1 06 The Periodic Table Sjp1618gcsechem



0 件のコメント:
コメントを投稿